Translate this page into:
When the Vitamin of Psychiatry Becomes the Cause of Misery: A Case Report on Clonazepam-Induced Hypersensitivity Reaction
* Corresponding author: Dr. Sudipta Borah MBBS, Department of Psychiatry, Jorhat Medical College and Hospital, Barbheta, Jorhat, India. borahsudipta1995@gmail.com
-
Received: ,
Accepted: ,
How to cite this article: Borah S, Bora D, Deka K. When the Vitamin of Psychiatry Becomes the Cause of Misery: A Case Report on Clonazepam-Induced Hypersensitivity Reaction. Acad Bull Ment Health. 2024;2:80-3. doi: 10.25259/ABMH_4_2024
Abstract
Clonazepam is the most widely prescribed anxiolytic in psychiatry, which is well tolerated and is rarely associated with cutaneous adverse drug reactions. This is a case of a 31-year-old male businessman diagnosed with generalized anxiety disorder (GAD), who later developed skin lesions after oral consumption of clonazepam. Dermatological consultation was sought, and the patient was diagnosed with a case of clonazepam-induced hypersensitivity reaction, corroborated by the blood investigations, the oral drug challenge test, and the histopathological skin biopsy report. The patient was managed with corticosteroids, anxiolytic drugs other than clonazepam, and a supportive approach. This is a unique case of a hypersensitivity reaction due to clonazepam, which will be helpful for future research and clinical practice.
Keywords
Clonazepam
Cutaneous adverse drug reaction
Hypersensitivity reaction
INTRODUCTION
Cutaneous Adverse Drug Reactions (CADR) can be caused by the use of psychotropic medications in some patients. The incidence of CADRs in patients using psychiatric drugs has been estimated to be approximately 2–5%, but in hospitalized patients, it is 1% to 3%.[1]
Clonazepam, approved in 1976 as an anti-epileptic drug by the United States Food and Drug Administration (USFDA), is the most commonly prescribed benzodiazepine for anxiety and panic disorders in day-to-day psychiatric practice.[2] It can be used to treat a wide range of psychiatric disorders, potentially earning it a role as a co-prescribed medication in numerous cases. Hence, clonazepam can also be loosely termed as “Vitamin of Psychiatry.”
Drowsiness and sedation are the common side effects of clonazepam. However, cutaneous side effects are not commonly seen. Some patients have reported rashes, dermatitis and allergic reactions, urticaria, angioedema, erythema multiforme as skin-related side effects of clonazepam.[1] Clinicians may not be able to recognize those side effects, probably due to the rarity of such incidents. Overall, the mortality rate of CADRs is 1.71%, but conditions like Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN) have a mortality rate as high as 16.39%.[3] Hence, early recognition of the CADRs is essential to prevent the severe unwanted consequences. Hereby, we report a case of GAD in a middle-aged man who presented to us with dermatological manifestations after oral administration of clonazepam.
CASE REPORT
A 31-year-old married businessman, hailing from a rural background in Jorhat, who is a case of GAD, attended the Psychiatry OPD in a tertiary care hospital in Assam, presenting with some itchy skin lesions mostly over his neck region and the limbs. History revealed that 5 days ago, he was prescribed the tablet Clonazepam 0.25 mg twice daily, along with Escitalopram 5 mg once daily, for his anxiety symptoms on his last visit to a physician in a private set-up. Two days later, he noticed skin lesions over his body which were progressively increasing, adding to his distress. History of recent intake of any other drug or psychoactive substance was ruled out. There was no history of bronchial asthma or any food or drug allergy. History of mental disorders in the family was absent.
On general examination, there was tachycardia and low-grade pyrexia, along with multiple erythematous plaques with vesicular skin eruptions over his bilateral upper and lower limbs and over his neck, upper trunk region, and abdomen [Figures 1a–1d]. There was no hypotension, lymphadenopathy, or signs of multiorgan damage. Mucosal or orogenital involvement was absent. Mental status examination of the patient revealed an anxious and agitated state, mostly concerned for his skin lesions. HAM-A scale was applied to measure the severity of anxiety symptoms, the total score of which was 30, indicating moderate anxiety. A dermatological consultation was taken for his skin manifestations. He was admitted in their department, and psychiatric follow-up was done on a regular basis. Initially, a drug-induced hypersensitivity reaction was strongly suspected, considering the offending drugs to be clonazepam and escitalopram. Hence, all the medications that he was consuming were withheld temporarily.Blood reports revealed high total count (15000/cu mm.) with normal eosinophil count (4%), slightly elevated Erythrocyte Sedimentation Rate (ESR) (30 mm at the end of first hour) and positive C-Reactive protein. However, to reconfirm our diagnosis, Oral Drug Challenge Test was performed under precautionary measures with clonazepam and escitalopram, one by one, after stopping them for 3 days. There was a reappearance of new skin lesions with exacerbation of the previous lesions on the same day, with tablet clonazepam 0.25 mg, but not with tablet escitalopram 2.5 mg.[4] The reaction was idiosyncratic. The histopathological findings of the skin biopsy from the right arm showed moderate perivascular infiltrates of lymphocytes along with few plasma cells and scattered eosinophils in the upper and mid-dermis, with a possibility of drug-induced changes. On the basis of the above findings, clonazepam-induced hypersensitivity reaction was confirmed. He was treated with intravenous dexamethasone, antibiotic coverage, oral antihistamines, and the local application of corticosteroids over the skin lesions. The patient got discharged and a tapered course of dexamethasone resulted in the gradual resolution of the rash over 2–3 weeks.

- Multiple erythematous plaques with vesicular skin eruptions over his bilateral upper and lower limbs, following clonazepam administration.

- Skin lesions over the ventral aspect of the upper arm.

- Dorsal and ventral aspects of the upper limb.
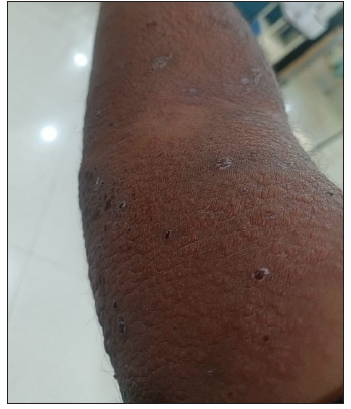
- Close view of the skin eruptions.
On discharge, for the psychiatric diagnosis of GAD, he was prescribed tablet Paroxetine 25 mg at bedtime and tablet Buspirone 5 mg twice daily. The patient was followed up after 2 weeks and then monthly, both in Psychiatry and Dermatology OPD, with a significant reduction in the initial symptoms. The skin lesions did not recur. We are in contact with the patient till date, who is regular with his prescribed medications, with no symptoms of anxiety or skin rash at present and is doing incredibly well in his routine activities.
DISCUSSION
Clonazepam is frequently recommended to treat a variety of psychiatric conditions such as anxiety disorders, acute stress reactions, sleep-related disorders, etc., and also as an augmenting agent to other psychotropics. Very few occurrences of cutaneous adverse effects, including lichenoid drug eruption, erythema multiforme, localized exfoliation eruptions, etc., have been documented in relation to clonazepam.[5-9] As per the reports, these rare dermatological side effects have been observed using dose ranges from 0.5 to 2 mg/day.
In our case, consideration of the time course of the illness in relation to drug administration was the most helpful approach in confirming the diagnosis – the temporal criteria for drug-induced hypersensitivity reaction were met in this case with respect to administration and withdrawal of clonazepam. A probability scale of adverse drug reaction, namely, the Naranjo algorithm, was applied, and the association between clonazepam and hypersensitivity reaction was found to be definite (a score of 9), whereas the score with escitalopram was 0, making its association doubtful as shown in Table 1.[10] The diagnosis was further supported by the oral drug challenge test, which played a significant role in identifying the offending agent and in excluding the other harmless but essential medications from being wrongly suspected as culprit. Furthermore, the histopathological findings of the skin biopsy, suggestive of a possibility of drug-induced changes, were the icing on the cake.
| Offending drug | Naranjo algorithm score | Interpretation |
|---|---|---|
| Clonazepam | 9 | Definite |
| Escitalopram | 0 | Doubtful |
CONCLUSION
The literature regarding the association of clonazepam and hypersensitivity reaction is very limited till date. This case report adds to the evidence that cutaneous adverse drug reactions such as erythematous vesicular lesions may be caused by clonazepam. Physicians should be aware of this and should take into account any previous history of CADR with clonazepam or other drugs belonging to the same group. In this regard, this case report will be very beneficial for further research and clinical vigilance.
Ethical approval
Institutional Review Board approval is not required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent.
Financial support and sponsorship
Nil
Conflicts of interest
Dr. Dipjyoti Bora and Dr.Kamala Deka are on the Editorial Board of the Journal.
Use of artificial intelligence (AI)-assisted technology for manuscript preparation
The authors confirm that there was no use of Artificial Intelligence (AI)-Assisted Technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
REFERENCES
- Critical Overview: Adverse Cutaneous Reactions to Psychotropic Medications. J Clin Psychiatry. 1999;60:3933.
- [PubMed] [Google Scholar]
- Indian Psychiatric Society Multicentric Study: Prescription Patterns of Psychotropics in India. Indian J Psychiatry. 2014;56:253.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Cutaneous Adverse Drug Reactions in Indian Population: A Systematic Review. Indian Dermatol Online J. 2014;5:S76-86.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Testing for Drug Hypersensitivity Syndromes. Clin Biochem Rev. 2013;34:15.
- [PubMed] [PubMed Central] [Google Scholar]
- Clonazepam-Induced Lichenoid Drug Eruption: A Case Report. BMC Psychiatry. 2021;21:1-4.
- [CrossRef] [PubMed] [PubMed Central] [Google Scholar]
- Erythema Multiforme Due to Clonazepam - Supportive Evidence from the Macrophage Migration Inhibition Factor Test. Clin Exp Dermatol. 1998;23:206-7.
- [CrossRef] [PubMed] [Google Scholar]
- Suspected Clonazepam-Induced Bullous Dermatosis in a Patient with Respiratory Failure. J Clin Pharmacol. 2012;52:1607-9.
- [CrossRef] [PubMed] [Google Scholar]
- Correlation between Serum TARC Levels and Eczematous Drug Eruption Following Oral Challenge Test with Clonazepam. Clin Exp Dermatol. 2020;45:1063-5.
- [CrossRef] [PubMed] [Google Scholar]
- Clonazepam-Induced Oral Lichenoid Reaction: A Case Report. New York State Dent J. 2020;86:24-7.
- [Google Scholar]
- Naranjo ADR Probability Scale. Clin Pharmacol Ther. 1981;30:239-45.
- [CrossRef] [PubMed] [Google Scholar]








